In the evolving landscape of neurodegenerative therapeutics, a novel approach targeting alpha-synuclein aggregation through nanobodies is garnering significant attention from researchers and clinicians alike. Alpha-synuclein, a presynaptic neuronal protein, is intrinsically involved in the pathogenesis of several disorders, most notably Parkinson's disease and other synucleinopathies. Its misfolding and subsequent aggregation into toxic oligomers and Lewy bodies are central to the disease progression, leading to neuronal dysfunction and cell death. Traditional therapeutic strategies have struggled to effectively intervene in this process, often due to challenges in specificity, delivery, and crossing the blood-brain barrier. This is where the unique properties of nanobodies offer a promising alternative.
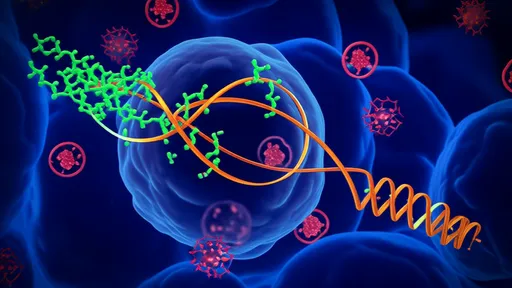
Nanobodies, derived from heavy-chain-only antibodies found in camelids, represent a groundbreaking class of biological agents. Their small size, exceptional stability, and high affinity for antigens make them ideally suited for targeting intricate pathological structures like protein aggregates. Unlike conventional antibodies, nanobodies can access epitopes that are often hidden or less accessible, providing a tactical advantage in disrupting the formation of alpha-synuclein fibrils. Recent studies have demonstrated that engineered nanobodies can not only bind selectively to oligomeric forms of alpha-synuclein but also inhibit their propagation between cells, a mechanism critical to the spread of pathology in the brain.
The design and selection of these nanobodies involve sophisticated techniques such as phage display and yeast surface display libraries, allowing researchers to identify variants with the highest specificity and neutralizing activity. Some of the most advanced candidates have shown an ability to recognize specific conformational epitopes on misfolded alpha-synuclein, thereby preventing further aggregation and promoting disassembly of existing aggregates. This targeted approach minimizes off-target effects, a common drawback of broader inhibitory strategies. Moreover, their modular nature allows for further engineering; for instance, they can be conjugated with molecules that facilitate blood-brain barrier penetration or linked to modalities that enhance clearance mechanisms.
Delivery remains a pivotal challenge in treating neurodegenerative diseases, but nanobodies possess inherent features that address this hurdle. Their compact structure improves tissue penetration and distribution within the central nervous system when administered via various routes, including intranasal and systemic delivery. Preclinical models have illustrated that nanobody-based therapeutics can significantly reduce alpha-synuclein burden in the brain, correlating with improved motor function and neuroprotection. These findings are bolstering confidence in their translational potential, with several candidates advancing toward early-phase clinical trials.
Beyond mere inhibition, some innovative approaches are exploring nanobodies as vehicles for targeted degradation of alpha-synuclein aggregates. By fusing nanobodies to components of the ubiquitin-proteasome system or autophagy pathways, researchers are creating degrader nanobodies that not only bind but also flag the pathological proteins for cellular disposal. This dual functionality represents a significant leap forward, moving from inhibition to active clearance, which could be crucial for reversing established pathology in later stages of disease.
However, the path to clinical application is not without obstacles. Immunogenicity, although reduced due to humanization techniques, requires careful monitoring. Long-term safety and the ability to sustain therapeutic effects are critical parameters under investigation. Additionally, the heterogeneity of alpha-synuclein aggregates across patients suggests that a one-size-fits-all nanobody might be insufficient; personalized or combination therapies could be necessary to address varied pathological profiles.
The economic and manufacturing aspects also play a role in the viability of nanobody therapies. While production is generally more cost-effective than for traditional antibodies, scaling up to meet clinical demand while maintaining quality control presents its own set of challenges. Regulatory agencies are closely watching these developments, with guidelines for biologic therapies continuously evolving to accommodate such innovative modalities.
In conclusion, nanobodies targeting alpha-synuclein aggregation stand at the forefront of a new era in neurodegenerative treatment. Their precision, versatility, and favorable pharmacokinetic profile position them as powerful tools against synucleinopathies. As research progresses, these molecules may not only offer hope for halting disease progression but potentially for reversing the damage wrought by these devastating conditions. The coming years will be pivotal in translating these promising preclinical results into tangible benefits for patients worldwide.
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025