In the intricate world of cellular biology, the ability of cells to withstand and adapt to stress is a fundamental survival mechanism. Among the key players in this defense system are heat shock proteins, with Heat Shock Protein 70 (HSP70) standing out as a central molecular chaperone. Its role in maintaining protein homeostasis, particularly under conditions of cellular stress such as heat, toxins, or disease, underscores its biological significance. This article delves into the molecular chaperone mechanisms of HSP70, exploring how it assists in protein folding, prevents aggregation, and facilitates the refolding or degradation of damaged proteins, thereby ensuring cellular integrity and function.
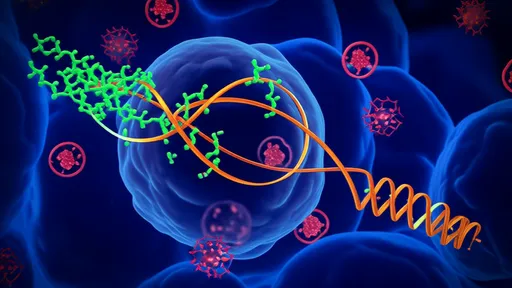
The structure of HSP70 is elegantly designed for its chaperone functions. It consists of two primary domains: a nucleotide-binding domain (NBD) and a substrate-binding domain (SBD). The NBD is responsible for ATP binding and hydrolysis, which drives the conformational changes essential for HSP70's activity. The SBD, in turn, interacts with client proteins, typically recognizing hydrophobic regions exposed in misfolded or unfolded polypeptides. This domain organization allows HSP70 to act as a dynamic machine, switching between high-affinity and low-affinity states for substrates based on its nucleotide status. When ATP is bound, the SBD has low affinity for substrates, promoting rapid binding and release. Hydrolysis of ATP to ADP induces a conformational shift that enhances substrate affinity, stabilizing the interaction and allowing time for proper folding or processing.
Central to HSP70's chaperone mechanism is its cooperation with co-chaperones, which regulate its ATPase activity and substrate binding. Among these, J-domain proteins (such as Hsp40 in humans) play a critical role by stimulating ATP hydrolysis, thereby facilitating the transition to the high-affinity ADP state. This interaction ensures that HSP70 engages substrates efficiently at the right time and place. Additionally, nucleotide exchange factors (e.g., BAG-1 or Hsp110) promote the release of ADP and binding of ATP, resetting the cycle for another round of chaperone activity. This regulated cycle allows HSP70 to perform diverse functions, from de novo protein folding to preventing aggregation under stress conditions.
Under cellular stress, such as thermal shock, proteins are prone to denaturation and misfolding, leading to potentially toxic aggregates. HSP70 is rapidly upregulated in response to such insults, acting as a first line of defense. It binds to exposed hydrophobic patches on misfolded proteins, shielding them from inappropriate interactions that could lead to aggregation. By maintaining these clients in a folding-competent state, HSP70 provides a window of opportunity for refolding once conditions normalize. In cases where refolding is not possible, HSP70 can also target damaged proteins for degradation via the ubiquitin-proteasome system or autophagy, thereby preventing the accumulation of toxic species that could compromise cell viability.
Beyond stress response, HSP70 is involved in a myriad of physiological processes. It assists in the translocation of proteins across membranes, such as into the mitochondria or endoplasmic reticulum, by maintaining precursors in an unfolded state competent for transport. In signaling pathways, HSP70 modulates the activity of key regulators, including kinases and transcription factors, influencing processes like apoptosis and inflammation. Its role in immunity is particularly noteworthy, as it can facilitate antigen presentation and modulate immune responses, making it a molecule of interest in therapeutic strategies for diseases ranging from cancer to neurodegenerative disorders.
The therapeutic potential of HSP70 is vast, given its central role in cellular protection. In cancer, HSP70 is often overexpressed, helping tumor cells survive hostile environments and resist chemotherapy. Inhibiting HSP70 has emerged as a strategy to sensitize cancer cells to treatment. Conversely, in neurodegenerative diseases like Alzheimer's or Parkinson's, where protein aggregation is a hallmark, enhancing HSP70 activity could promote clearance of toxic aggregates and protect neurons. However, manipulating HSP70 is challenging due to its ubiquitous functions and complex regulation, requiring targeted approaches to avoid off-target effects. Research into small molecule modulators and gene therapies continues to advance, holding promise for future applications.
In summary, Heat Shock Protein 70 exemplifies the sophistication of cellular stress management through its molecular chaperone mechanisms. Its ability to bind, fold, and decide the fate of proteins under duress is a testament to the elegance of evolutionary adaptation. As research unravels more details of its interactions and functions, the potential to harness HSP70 for therapeutic interventions grows, offering hope for addressing some of the most challenging diseases in modern medicine. The journey from basic understanding to clinical application remains long, but the foundational role of HSP70 in cellular health makes it a compelling focus for continued exploration.
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025