In the intricate tapestry of nature's collaborations, few relationships are as fascinating and industrially significant as the symbiotic system within the termite gut. These often-maligned insects, frequently cast as villains for their wood-devouring habits, are in fact marvels of biological engineering, operating not alone, but as a complex superorganism. Their ability to break down the formidable compound lignin, a key component of wood, is not an innate talent but a power granted to them by a diverse and highly specialized community of microbial partners residing in their digestive tracts.
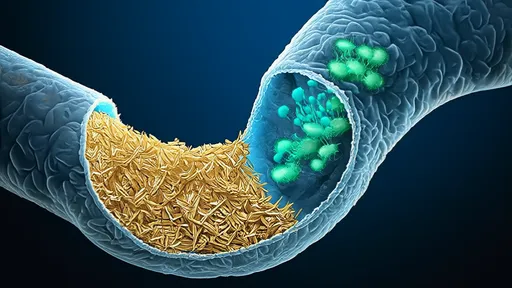
The central challenge of consuming wood lies in its composition. Plant cell walls are fortified by lignin, a complex and recalcitrant phenolic polymer that acts as a natural shield, providing structural support and protecting the more digestible cellulose and hemicellulose from attack. For most animals, lignin is indigestible; it is the reason we cannot derive nutrition from a handful of sawdust. Termites, however, have evolved a solution that has eluded human scientists for decades: a sophisticated, internal bioreactor that efficiently deconstructs this stubborn material.
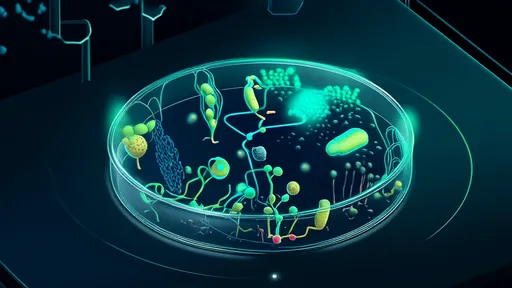
This process unfolds within the termite's hindgut, a specialized chamber that has become a hospitable home for a stunning array of protozoa, bacteria, and archaea. This is not a simple partnership but a multi-layered symbiosis. The termite provides a stable, anaerobic environment and a constant supply of finely chewed wood particles. In return, the microbial consortium undertakes the arduous task of digestion. The protozoa, often unique to termites, are the first line of attack. They engulf the wood particles and initiate the breakdown process using their own enzymes. Following this, a diverse suite of bacteria takes over, further processing the fragments and metabolizing the products into simpler compounds.
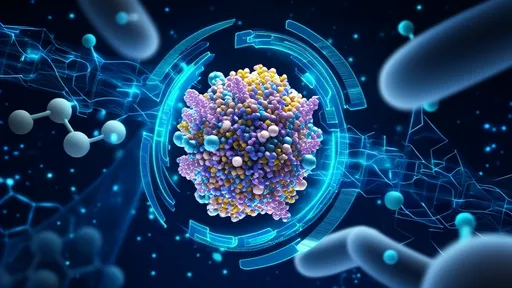
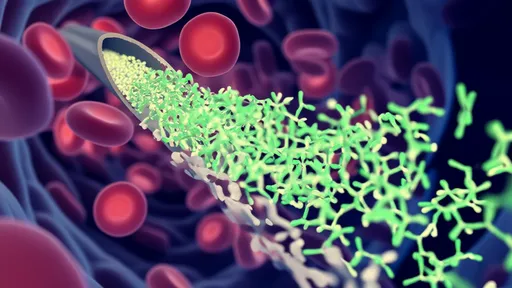
The true magic lies in the enzymatic arsenal of these microbes. They produce a vast array of specialized enzymes, including lignin-modifying enzymes like laccases and peroxidases, as well as a full suite of cellulases and hemicellulases. These enzymes work in a coordinated cascade, first disrupting the lignin matrix to "unlock" the cellulose and hemicellulose fibers, and then systematically breaking these polysaccharides down into soluble sugars. The final products of this communal effort—short-chain fatty acids like acetate—are then absorbed by the termite and serve as its primary energy source. In essence, the termite farms its microbes for fuel.
The implications of understanding this system extend far beyond entomological curiosity. In a world urgently seeking sustainable alternatives to fossil fuels, the termite gut microbiome presents a blueprint for advanced biorefining. The industrial process of converting plant biomass (lignocellulose) into biofuels like ethanol is currently hampered by a costly and energy-intensive pretreatment step required to overcome the lignin barrier. Termites and their microbes perform this step with stunning efficiency at ambient temperature and pressure. By studying and harnessing the enzymes and microbial pathways from the termite gut, scientists aim to develop more efficient and economical biological pretreatments, potentially revolutionizing the biofuel industry and paving the way for a truly circular bioeconomy.
Furthermore, this research holds promise for waste management. The ability to break down woody debris and agricultural waste, such as corn stover and sugarcane bagasse, could transform these abundant byproducts from waste streams into valuable resources for energy and chemical production. The termite, therefore, is more than a pest; it is a keyholder to a treasure trove of biochemical innovation, offering elegant biological solutions to some of our most pressing industrial and environmental challenges. Its gut is a testament to the power of collaboration, reminding us that some of nature's most powerful technologies are hidden in plain sight, operating on a microscopic scale within the most unexpected of creatures.
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025