The landscape of regenerative medicine is undergoing a profound transformation, driven by a paradigm shift away from traditional stem cell approaches toward the more direct and revolutionary technique of cellular transdifferentiation. At the forefront of this exciting field is the direct conversion of fibroblasts, the most common cells in connective tissue, into functional hepatocytes, the primary functional cells of the liver. This process bypasses the pluripotent stem cell stage entirely, offering a faster, potentially safer, and more efficient route to generating the vast quantities of hepatic cells needed for therapy, disease modeling, and drug screening.
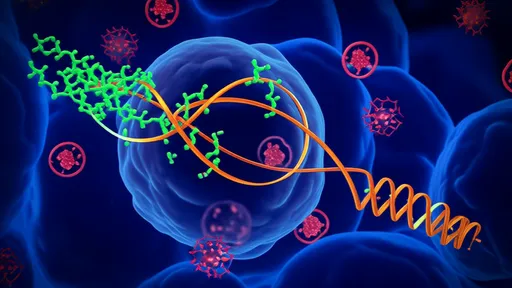
The scientific principle underpinning this conversion is the forced reprogramming of a cell's identity. Fibroblasts and hepatocytes, though both originating from the same embryonic layer, possess vastly different gene expression profiles and functions. The key to transdifferentiation lies in identifying and activating the master regulatory genes that define a hepatocyte while simultaneously suppressing the fibroblast-specific genetic program. Researchers achieve this primarily through the targeted delivery of specific transcription factors—proteins that bind to DNA and control the flow of genetic information. Pioneering studies identified a core cocktail of factors, such as HNF1α, HNF4α, and FOXA3, whose introduction into fibroblasts via viral vectors can initiate a dramatic molecular metamorphosis, steering the cell toward a hepatic fate.
The implications of successfully generating induced hepatocytes (iHeps) through transdifferentiation are monumental for treating liver disease. The liver possesses a remarkable innate capacity for regeneration; however, this ability is overwhelmed in cases of acute liver failure, end-stage cirrhosis, or metabolic liver diseases. Liver transplantation remains the only curative option, but it is severely limited by a critical shortage of donor organs. Transdifferentiation offers a beacon of hope, proposing a future where a patient's own skin fibroblasts could be harvested, reprogrammed into healthy iHeps, and then transplanted back into the damaged liver. This autologous approach would eliminate the risk of immune rejection and the lifelong need for immunosuppressant drugs, hurdles that plague both organ transplantation and therapies derived from donor stem cells.
Beyond direct cell therapy, the technology opens new frontiers in pharmaceutical research and toxicology. The pharmaceutical industry invests immense resources into drug development, only to see many promising compounds fail in late-stage clinical trials due to unforeseen hepatotoxicity—liver damage. A primary reason for these failures is the reliance on imperfect models, such as animal liver cells or cancerous human cell lines, which often do not accurately mimic the complex metabolic functions of a healthy human hepatocyte. Transdifferentiated iHeps, especially when derived from human fibroblasts, provide a limitless source of physiologically relevant, non-cancerous human hepatocytes. These cells can be used to create more accurate in vitro models and high-throughput screening platforms, enabling researchers to identify toxic side effects much earlier in the drug development pipeline, thereby saving billions of dollars and improving patient safety.
Furthermore, this technology empowers the creation of personalized in vitro models for a new era of precision medicine. By generating iHeps from fibroblasts taken from patients with specific genetic liver disorders—such as Alpha-1 Antitrypsin Deficiency, Wilson's disease, or familial hypercholesterolemia—scientists can now cultivate a living, human model of that patient's disease in a petri dish. These patient-specific disease models are invaluable tools for unraveling the underlying molecular mechanisms of the pathology and for screening vast libraries of compounds to find drugs that could potentially correct the specific defect in that individual's cells. This approach moves us closer to tailored therapeutic strategies that are uniquely effective for each patient's genetic makeup.
Despite the tremendous promise, the path from laboratory breakthrough to clinical application is fraught with significant challenges that the scientific community is actively working to address. A primary concern is the efficiency and completeness of the conversion process. Not all fibroblasts within a population successfully reprogram into fully functional iHeps, and the resulting cells can sometimes exhibit immature or unstable characteristics. Current methods often rely on viral vectors to deliver the reprogramming factors, which raises safety concerns regarding insertional mutagenesis—where the viral DNA disrupts an important gene and potentially triggers cancer. Intensive research is now focused on developing non-integrating delivery methods, such as Sendai virus, mRNA, or CRISPR-based activation systems, to mitigate this risk and pave the way for clinical use.
Looking ahead, the future of fibroblast-to-hepatocyte transdifferentiation is incredibly bright. Research is increasingly focused on refining the protocol to enhance the maturity, functionality, and long-term stability of iHeps to match that of primary adult hepatocytes. Scientists are exploring the role of the cellular microenvironment, or niche, by investigating how synthetic scaffolds and co-culture with other cell types can promote more robust maturation and function. The ultimate goal is to scale up the production of clinical-grade iHeps under strict Good Manufacturing Practice (GMP) conditions, making this transformative therapy accessible. As these technical hurdles are overcome, we stand on the cusp of a new chapter in medicine, where creating custom-made, healthy human cells from a simple skin biopsy becomes a standard and life-saving procedure.
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025