The landscape of regenerative medicine has been fundamentally reshaped by the advent of cellular reprogramming, with somatic cell transdifferentiation emerging as a particularly promising frontier. Unlike the generation of induced pluripotent stem cells (iPSCs), which involves a complete reversion to a pluripotent state, transdifferentiation offers a more direct route. It coerces one mature, differentiated somatic cell to transform directly into another distinct somatic cell type, bypassing the pluripotent intermediate. This elegant shortcut is not merely a scientific curiosity; it is rapidly maturing into a platform with profound and tangible clinical applications, heralding a new era in patient-specific therapies.
The clinical allure of transdifferentiation lies in its inherent advantages. By sidestepping the pluripotent stage, the technique theoretically mitigates the significant risk of teratoma formation associated with residual undifferentiated iPSCs. Furthermore, the process can be more efficient, requiring less time to generate the desired functional cells. This direct conversion also often better preserves the epigenetic age and functionality of the original cell, potentially yielding more mature and clinically relevant tissues. The goal is no longer just to create cells in a dish, but to engineer robust, transplantable units that can seamlessly integrate into a patient's body to repair damage and restore function.
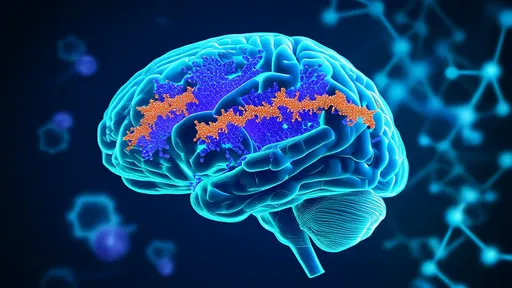
One of the most vibrant areas of clinical translation is in neurology. Researchers are making significant strides in converting human fibroblasts directly into induced neurons (iNs). These are not mere facsimiles; they exhibit electrical activity and can form functional synapses. The implications for diseases like Parkinson's are staggering. Imagine a future where a small skin biopsy from a patient is taken, the fibroblasts within are reprogrammed directly into dopaminergic neurons, and these patient-specific neurons are then transplanted back into the brain to replace those lost to the disease. This approach eliminates the need for immunosuppression and the ethical quandaries of fetal tissue use, presenting a truly personalized therapeutic strategy. Similar efforts are underway for Alzheimer's disease, Huntington's disease, and spinal cord injuries, aiming to rebuild shattered neural circuits from within.
Cardiovascular medicine stands to be another major beneficiary. The heart possesses limited innate regenerative capacity, making damage from myocardial infarction often permanent. Transdifferentiation offers a solution by enabling the generation of new cardiomyocytes directly. Scientists have successfully reprogrammed cardiac fibroblasts, the cells that form scar tissue after a heart attack, into functional, beating cardiomyocytes in vivo. This concept—turning the damaging scar-forming cells into functional heart muscle cells—represents a paradigm shift in cardiac repair. Early-stage clinical explorations are focusing on delivering reprogramming factors directly to the heart tissue to catalyze this healing process from within, potentially reversing the damage caused by heart failure.
The field of diabetes treatment is also undergoing a revolution fueled by this technology. The primary objective is to generate glucose-responsive, insulin-secreting beta cells to replace the ones destroyed by the autoimmune attack in Type 1 diabetes. Pioneering work has demonstrated the feasibility of transdifferentiating a patient's own cells, such as hepatocytes or exocrine pancreatic cells, into functional beta-like cells. These cells can sense glucose levels and secrete insulin in a regulated manner. Autologous transplantation of these lab-grown islet cells could restore natural insulin production, freeing patients from lifelong insulin injections and the constant fear of hypoglycemic events. This approach promises a functional cure, not just a management strategy.
Despite the exhilarating progress, the path to the clinic is paved with formidable challenges. A primary concern is the efficiency and purity of the conversion process. Current protocols often yield a mixed population of cells, and the presence of imperfectly reprogrammed or residual original cells could lead to unintended consequences or poor graft function. Ensuring that every converted cell is fully functional and safe for transplantation is paramount. Additionally, most methods still rely on the use of viral vectors to deliver the reprogramming factors, raising concerns about insertional mutagenesis and long-term genetic stability. The development of non-integrating methods, such as using messenger RNA or small molecules, is a critical area of intense research to ensure the ultimate safety of these therapies.
Looking ahead, the future of transdifferentiation is incredibly bright. Research is pushing beyond these initial applications to explore its potential in generating hepatocytes for liver failure, keratinocytes for burn victims, and retinal pigment epithelial cells for macular degeneration. The concept of in vivo reprogramming—directly converting cells within the body without ever removing them—is particularly compelling. This would effectively allow us to instruct our bodies to heal themselves using their own cellular resources. As we refine the precision and safety of these techniques, we move closer to a new medical paradigm where degenerative diseases are not managed but cured, and organ damage is not endured but repaired.
In conclusion, somatic cell transdifferentiation has boldly moved from a provocative concept in a developmental biology textbook to a disruptive force in clinical medicine. It embodies the shift towards truly personalized, regenerative therapies. While significant hurdles in safety and manufacturing scalability remain, the progress is undeniable. This technology holds the promise of unlocking the body's latent potential for repair, offering hope for millions of patients awaiting cures for some of humanity's most debilitating conditions. The journey from skin cell to neuron, from fibroblast to cardiomyocyte, is no longer science fiction; it is the thrilling reality of modern medicine's next chapter.

By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025
By /Aug 25, 2025
By /Aug 25, 2025
By /Aug 25, 2025
By /Aug 25, 2025
By /Aug 25, 2025
By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025